Partnership
Microsoft GxP Compliance
Helping life sciences companies achieve high-impact business outcomes with Microsoft & USDM's GxP compliance
Microsoft empowers life science companies to reimagine ways to bring together people, data, and processes to advance scientific innovation, market products faster, enable virtual teams’ success, and improve operational and patient outcomes. Microsoft and USDM Life Sciences enable regulated businesses to transform their GxP workflows into the cloud with USDM’s Cloud Assurance for the Microsoft Azure Platform.
Your business depends on every layer of your technology to comply with FDA and global regulations for software assurance. From your infrastructure to data centers to the platform layers and many business applications that power your productivity, Microsoft and USDM can ensure your operation is continuously compliant.
Read our case study to learn how USDM helped a Top 5 global pharmaceutical company create a validated Artificial Intelligence (AI) framework for Microsoft Azure.
USDM's Approach
Our approach to regulated workloads in the public cloud covers end-to-end technology solutions to transform your regulatory operations from a cost-center to an innovation hub. Our building block delivery model addresses SaaS (business applications), PaaS (cloud services), and IaaS (global infrastructure).
The image below shows how Cloud Assurance supports the compliance requirements of your Azure infrastructure and Microsoft cloud applications with USDM’s building block approach.
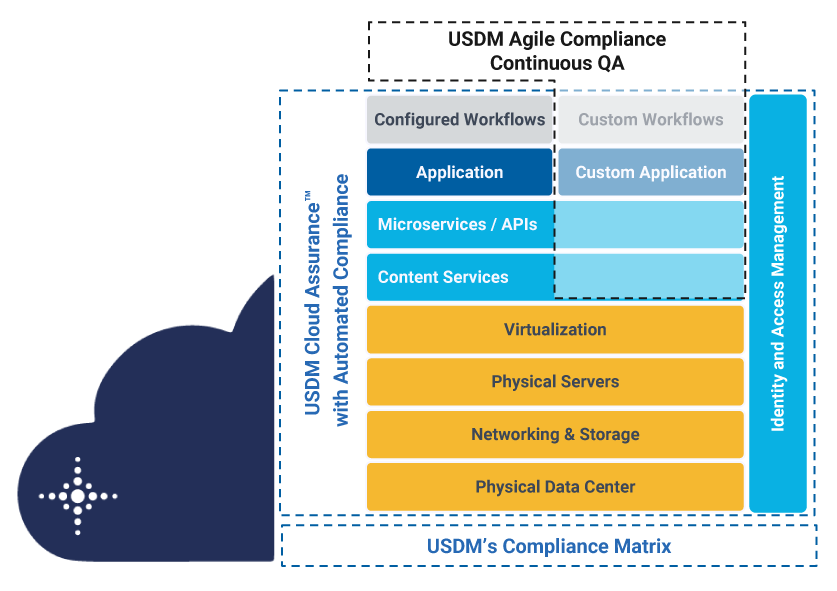
USDM Cloud Assurance for Microsoft Azure - Microsoft GxP Compliance Preferred Solution
USDM enables pharmaceutical, biotechnology, and medical device companies to transform their GxP workflows with Cloud Assurance for Azure. The solution addresses SaaS (business applications), PaaS (cloud services), and IaaS (global infrastructure). All packaged in USDM’s Cloud Assurance subscription to manage ongoing updates and decrease your compliance burdens. USDM Cloud Assurance for Microsoft Azure is the Microsoft GxP compliance preferred solution.
FEATURES
Cloud Assurance provides expert compliance services, fast-start accelerators, and defensible validation deliverables based on 20+ years of life sciences solution delivery.
- Expert Compliance Services – an Annual Vendor Audit, Infrastructure Qualification, Cloud Services Qualification, Cloud Application Validation, and an IS Health Check
- Fast-Start Accelerators – Impact Assessments, Procedures & Controls, Change Management, and Qualification Package
- Defensible Validation Deliverables – Qualification(s), Qualification Plan (System Requirements, Configuration Specification, CQ/PQ, Qualification Report), USDM’s Assurance Report and Vendor Certification
BENEFITS
By leveraging USDM’s life sciences expertise and accelerated cloud services, you can rapidly implement a GxP compliant framework.
- Reduce your compliance risk and achieve continuous cloud compliance with USDM’s framework and accelerators for Azure
- Speed deployment and adoption of Azure and Microsoft business applications with prepackaged solutions built-on life sciences best practices
- Accelerate your cloud journey by identifying strategic business initiatives made possible with Azure
- Flexible cloud adoption model to migrate regulated workloads
- Cost-effective, bundled solution to minimize barriers to compliance and innovation
- Build value-creating activities, decrease value-consuming activities
To learn more about Cloud Assurance for Microsoft Azure, visit the Azure Marketplace.
USDM Cloud Assurance for SharePoint - Microsoft GxP Compliance Preferred Solution
Rapid implementation, validation, and maintenance to achieve continuous GxP compliance & leverage your IT investment for GxP compliant content management
USDM can optimize your existing SharePoint Online application to include the management of GxP regulated content. By utilizing USDM’s fast-start tools and accelerators, USDM will significantly decrease your implementation and validation efforts of SharePoint Online. Further, USDM’s Cloud Assurance subscription will maintain compliance with all your SharePoint Online releases, patches, and changes so you can keep innovating in a continuously compliant state.
SUBSCRIPTION FEATURES
- Delivered for Computer System Validation (CSV) or Computer Software Assurance (CSA)
- Initial Validation Accelerators include a defensible annual vendor audit for the FDA, Part 11 and EU Annex 11 Assessment, Validation Plan, URS/FSR, IQ/OQ/PQ Protocol and Test Scripts, Traceability Matrix, and Validation Summary Report
- Cloud Assurance Maintenance includes vendor release management, impact assessments, updated validation documents, test execution for core releases, analysis, and reporting
BENEFITS
- Reduce your risk and achieve continuous cloud compliance with USDM’s accelerators and framework for SharePoint
- Fast-track your implementation with industry experts who can guide you in pre-implementation work through end-user training to increase adoption
- Accelerate your cloud journey by starting with SharePoint and making it possible to achieve a GxP compliant Azure tech-stack
- Leverage USDM’s comprehensive, defensible annual vendor audit for your SharePoint qualification
- Develop your business content governance structure and efficiently map to SharePoint workflows
To learn how USDM can support your entire Azure tech-stack, see USDM’s Unify Public Cloud for Azure subscription offering.
USDM Cloud Assurance for SharePoint M365
Leverage your IT investment for GxP-compliant content management
USDM can optimize your existing SharePoint M365 application to include the management of GxP-regulated content. By utilizing our best practices, we will significantly decrease your implementation and validation efforts of SharePoint M365. Further, USDM’s Cloud Assurance managed subscription will maintain compliance with all your SharePoint M365 releases so you can keep innovating in a continuously compliant state.
Watch our on-demand webinar: Accelerate Your Journey to the Cloud: Move your GxP Regulated Workloads to Microsoft Azure
How USDM Cloud Assurance for SharePoint M365 can support your business process:
- Fast-track your implementation with project management experts who can guide you in pre-implementation work through end-user training
- Provide your organization with a comprehensive Vendor Assurance Report to save time and effort with your vendor requalification endeavors
- USDM best practice Configuration Specification for Part 11 / Annex 11 compliance allows you to rapidly configure SharePoint M365 E5 for site collection, secure with E5 Azure Active Directory, and use DocuSign for workflows and electronic signatures
- USDM automated verification and qualification testing relieve your testing effort while providing the documented summary and traceability evidence you need for compliance
- Utilize our SharePoint M365 Standard Operating Procedures (SOPs) to ensure tasks are performed according to approved procedures
- USDM Cloud Assurance ensures your validated SharePoint M365 environment maintains a continuous state of compliance through all releases, patches, and changes
- Develop your business content governance structure and efficiently map to SharePoint M365 workflows
- Significantly reduce implementation and validation time while decreasing the cost to manage cloud compliance
- Leverage critical organizational change management, training, and communications support to ensure your IT investments are successfully adopted
USDM can accelerate your business transformation from trees to technology using SharePoint M365 in a GxP-compliant way! Read our blog, Validating SharePoint for Life Sciences Regulated Environments, for more information.
Learn more about our compliance services for emerging and start-up life sciences companies here.
Contact us to get started today!
Additional SharePoint Resources
Interested in becoming Cloud Assurance Certified?


