Learn why defining and measuring value helps to provide a trusted solution that responds to changes and scales to meet your needs.
What are traditional managed services? They’re often successful efforts to achieve year-over-year savings for customers, but there isn’t always a tangible connection between outcomes and the value they generate.
USDM Life Sciences believes that successful outcomes are the proof that a managed service like integrated GxP compliance generates value for your organization.
Illustrating Transformational Outcomes
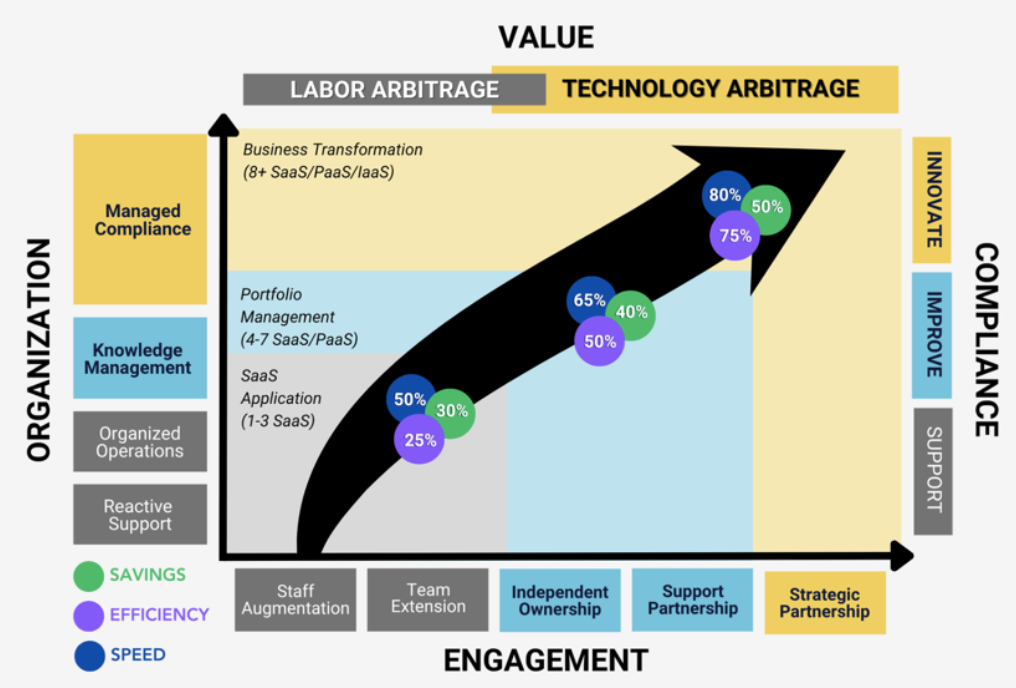
This value model for labor and technology arbitrage is based on organizational processes, engagement relationships, and compliance maturity. As shown in this graphic, when your portfolio reaches eight or more Software-as-a-Service (SaaS) applications, you start to see greater cost savings (around 50%), organizational efficiency (around 75%), and speed of engagement (around 80%).
These estimates are based on actual data from more than 300 USDM Cloud Assurance subscribers.
As your GxP tech stack grows, interactions between IT systems become increasingly complex. Disconnected GxP systems create gaps in regulated business processes and no longer provide insights that could drive smarter, data-driven decisions.
GxP technology investments—including automated workflows and testing—should support your business goals. This level of planning, measuring, and monitoring helps to ensure the best possible outcomes for your organization.
Learn how integrated GxP compliance is driving value generation—download the white paper
Defining and Measuring Value
Integrated GxP compliance embraces the philosophy that managed services should be tailored to customer needs. It challenges traditional managed services models with its ability to scale and respond to change. The USDM Integrated GxP Compliance managed service framework is built upon two pillars: defining value and measuring value.
Defining value starts with aligning managed services with your organization’s strategic objectives. From there, we help you to:
- Link value to business outcomes like expediting time to market
- Nurture innovation using advanced technologies and automation
- Mitigate compliance-related risks that could tarnish your reputation
- Collaborate to implement insights, custom solutions, and domain expertise
Measuring value gives you metrics for success; for example, optimizing resources and reducing expenditures. The value of integrated GxP compliance can also be measured through:
- Tangible return on investment (ROI) like financial gains and intangible enhancements like brand value
- Operational agility that helps you respond to market dynamics and simplify compliance in regulated environments
- Strategic positioning as indicated by company reputation, market share, and partnerships
- Faster time to market that helps your organization gain a competitive edge
- Overall improvement in risk mitigation for compliance, security, and data integrity issues
- Novel product concepts and innovation achieved with artificial intelligence (AI), augmented reality, and virtual reality
- Continuous improvement and compliance proven through sustained value creation and regulatory audit success rates
- Greater customer and employee satisfaction because GxP systems perform better and are more reliable
The USDM Integrated GxP Compliance managed service includes quarterly business reviews to track your organization’s progress with new technologies and to keep you moving toward your expected outcomes.
Supporting Integrated GxP Compliance with Digital Quality
What is digital quality? It ensures that data is accurate, consistent, and retrievable throughout its lifecycle. In the life sciences industry, technology can be used in quality assurance and quality control processes to enhance digital quality.
The life sciences industry continues to see successful digital transformation. Technologies like cloud computing, the Internet of Things (IoT), and AI improve the efficiency, effectiveness, and reliability of your products and services. As innovation challenges the traditional quality function, organizations need to be familiar with processes and tools for:
- Automated test platforms to streamline change management using automated test suites instead of a team of reviewers. Within hours, an upgrade can be assessed and ready to release or sent back to development.
- Automated quality control that uses technologies like machine learning and robotics to automate repetitive tasks, reduce human error, and improve consistency.
- Real-time monitoring to track processes using IoT devices and sensors that provide instant feedback and adjustments.
- Predictive analytics to help anticipate potential quality issues through data analytics and machine learning algorithms and empower companies to take proactive measures.
Transitioning from Traditional to Digital Quality
The USDM Integrated GxP Compliance managed service supports the transition from traditional paper-based quality to digital quality. Digital tools and systems used in the quality process are reliable, secure, and compliant with regulations. Systems and vendors are audited to ensure that they comply with data integrity standards, cybersecurity measures, privacy, and digital-specific regulations. Quality leaders are equipped to integrate technologies that align digital transformation with the organization’s quality objectives.
USDM has strategies and solutions to help your organization achieve digital quality, including:
- Audit-as-a-Service to assess vendors and previous vendor audits. We help to develop service level agreements (SLAs) and standardize processes.
- Gap analysis and remediation to identify gaps and risks in your systems and processes. We deliver a report with our findings and provide recommendations for remediation.
- GxP Landing Zone to prepare your Google Cloud tech stack for regulated and non-regulated use. It’s purpose-built for life sciences and meets requirements for 21 CFR Part 11, Annex 11, and relevant GxP requirements.
How USDM Can Help
With more than 23 years of experience in the life sciences industry, our expertise is why companies trust us to achieve their quality and cost objectives. USDM Integrated GxP Compliance is more than a platform—it’s a promise. A promise to accelerate your journey from molecule to market. Contact us to get started.