Technical considerations and guidance for managing UDI data to meet the deadline
On 23 January 2024, the European Union published the latest regulation, “amending Regulations (EU) 2017/745 [MDR] and (EU) 2017/746 [IVDR] as regards a gradual roll-out of Eudamed [European Database on Medical Devices], information obligation in case of interruption of supply and transitional provisions for certain in vitro diagnostic medical devices.
Among the many changes to the Medical Devices Regulation (MDR) and In Vitro Diagnostic medical devices Regulation (IVDR), one of the most important is “to enable a gradual roll-out of the [EUDAMED modules] . . . that are finalised, instead of deferring the mandatory use of EUDAMED until the last of the six modules is completed.” The Regulation will “. . . enable a gradual implementation of individual EUDAMED modules once they have been audited and declared functional.”
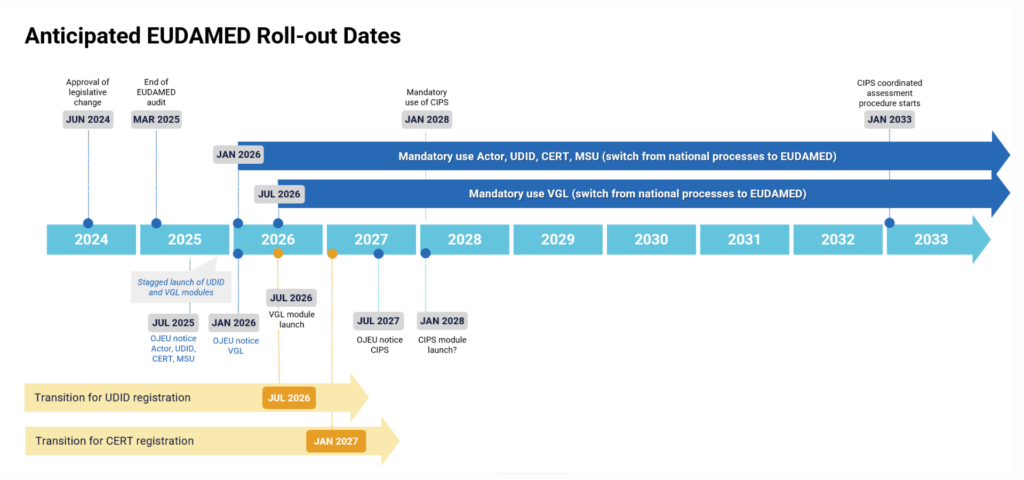
The European Commission published an updated timeline for the mandatory implementation of EUDAMED (below). Where the previous EUDAMED timeline showed mandatory use starting in Q2 2029, the new timeline shows the mandatory use of unique device identification (UDI) and device registration starting from Q1 2026.
Watch this important on-demand webinar, co-hosted by USDM Life Sciences and BAYARD, to understand the requirements and regulatory implications for your business. You’ll learn more about:
- Overview of EUDAMED and Data Management
- Overview of New Amendment and Corresponding Timelines
- Review of MDR, Legacy, and Old (NRD – not to be registered devices)
- Other Regional Updates (UK and Swiss)
- Sustainability
- Supply Chain Disruptions
- Connection Options
- Q&A
Questions and answers are at the end of the presentation.
About the Presenters
Jay Crowley, Vice President of Medical Device Solutions and Services USDM Life Sciences
At USDM, Jay provides business process, technology, and compliance solutions for the life sciences industry, and consults with medical device manufacturers to help them achieve regulatory compliance and a competitive advantage with UDI implementation. Previously, Jay was Senior Advisor for Patient Safety in the Food and Drug Administration’s Center for Devices and Radiological Health. He developed the framework and authored key requirements for the FDA’s Unique Device Identification system.
Lionel Tussau, Lead Healthcare, BAYARD
Lionel is responsible for developing healthcare activities at Bayard Consulting, including supporting manufacturers to register UDI data into regulatory databases like EUDAMED or the Global Unique Device Identification Database (GUDID), sharing sustainability and product information with trading partners, and leveraging the GS1 Global Data Synchronisation Network (GS1 GDSN). He represents Bayard in MedTech Europe (chairman of the EUDAMED IT Expert group), is an observer in the EUDAMED Medical Device Coordination Group (MDCG) working group, and is a GS1 Global Healthcare Leadership Team member.