Partners
Microsoft GxP Compliance
Bring together data, people, and processes to advance scientific innovation, get products to market faster, support virtual teams, and improve patient outcomes. Learn how the Microsoft + USDM partnership supports those endeavors.
Every layer of your technology—the data centers, infrastructure, platform, and business applications that power your productivity—must comply with global regulations.
USDM Cloud Assurance for Microsoft Azure is the Microsoft GxP compliance preferred solution.
USDM’s delivery model addresses SaaS (business applications), PaaS (cloud services), and IaaS (global infrastructure). This image shows how Cloud Assurance supports the compliance requirements of your Azure infrastructure and Microsoft cloud applications with a building-block approach.
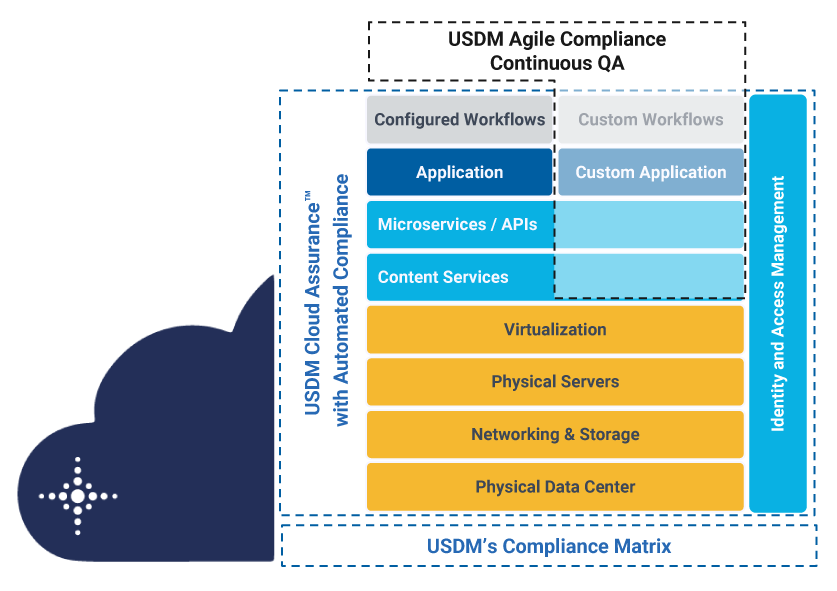
Incorporating more than 25 years of life sciences domain expertise, Cloud Assurance provides:
- Expert Compliance Services: an annual vendor audit, infrastructure qualification, cloud services qualification, cloud application validation, and an IS health check.
- Fast-Start Accelerators: impact assessments, procedures and controls, change management, and qualification package.
- Defensible Validation Deliverables: configuration and performance qualification (CQ/PQ), qualification plan (system requirements, configuration specification, qualification report), and USDM’s vendor certification and Vendor Assurance Report.
Leverage USDM’s cloud services to implement a GxP compliant framework that helps your organization:
- Reduce compliance risk and achieve continuous cloud compliance with accelerators for Azure
- Speed deployment and adoption of Azure and Microsoft business applications with prepackaged solutions built-on life sciences best practices
- Identify strategic business initiatives made possible with Azure
- Migrate regulated workloads with a flexible cloud adoption model
- Minimize barriers to compliance and innovation with a cost-effective solution
Learn how USDM helped a Top 5 global pharmaceutical company create a validated Artificial Intelligence (AI) framework for Microsoft Azure.
For more information about Cloud Assurance for Microsoft Azure, visit the Azure Marketplace.
USDM Cloud Assurance for SharePoint Online and SharePoint in Microsoft 365
USDM optimizes SharePoint to include GxP regulated content management. Using fast-start tools and accelerators, USDM significantly decreases your implementation and validation efforts. Further, USDM’s Cloud Assurance subscription maintains compliance after all releases, patches, and changes.
- Cloud Assurance delivered for Computer System Validation (CSV) or Computer Software Assurance (CSA).
- Initial validation accelerators include: a defensible annual vendor audit for the U.S. Food and Drug Administration (FDA), Part 11 and EU Annex 11 assessment, validation plan, URS/FSR, IQ/OQ/PQ protocol and test scripts, traceability matrix, and validation summary report.
- Cloud Assurance maintenance includes: vendor release management, impact assessments, updated validation documents, test execution for core releases, analysis, and reporting.
- Configuration specification for Part 11 and Annex 11 compliance and use DocuSign for workflows and electronic signatures.
- USDM’s SharePoint in Microsoft 365 standard operating procedures (SOPs) ensure tasks are performed according to approved procedures.
From pre-implementation work through end-user training, USDM makes it possible to achieve a GxP compliant Azure tech-stack.
Watch our on-demand webinar: Accelerate Your Journey to the Cloud: Move your GxP Regulated Workloads to Microsoft Azure
Additional Document and Content Management Capabilities
USDM is committed to delivering comprehensive solutions that integrate compliance, data management, automation, and content management to support the life sciences industry. Efficient document management ensures that content is compliant, organized, and easily accessible throughout its lifecycle.
Document and content management capabilities at USDM address:
- Content Readiness for GenAI: Prepare content for generative AI tools that produce automated insights. USDM helps your organization format and organize content for advanced analysis and AI-driven applications.
- Document Control and Lifecycle Management: Ensure that the document lifecycle—creating, approving, storing, and disposal—maintains content integrity. USDM helps you implement compliant lifecycle management.
- Compliance for Unstructured Data: Prevent compliance risks by handling unstructured data—such as emails, images, and documents—according to standards like 21 CFR Part 11. USDM helps you maintain operational transparency.
- Metadata Management and Searchability: Add metadata to improve document searchability and discoverability. USDM helps you establish effective metadata management that is scalable to large volumes of content.
- Secure Collaboration and Sharing: Share information with internal teams, regulators, and partners securely and compliantly. USDM helps you protect sensitive documents while facilitating collaboration.
Learn how USDM supports pre-commercial and emerging companies here.
Contact us today to get started!
Additional SharePoint Resources
Interested in becoming Cloud Assurance Certified?


