How USDM Life Sciences and Oracle are revolutionizing Enterprise Trace Matrix management through AI-powered compliance embedded directly in business workflows.
Every year, life sciences companies face the same sobering reality: FDA 483 audit observations consistently highlight the same top compliance failures across the industry despite decades of traditional compliance approaches.
- Inadequate change control and impact analysis
- Failure to follow standard operating procedures (SOPs)
- Inadequate training programs
- Inadequate documentation and record-keeping
The root cause? Manual, fragmented Enterprise Trace Matrix (ETM) management that simply cannot keep pace with the dynamic nature of modern regulatory environments.
USDM Life Sciences in partnership with Oracle is introducing a fundamental shift in how life sciences organizations can approach compliance management. ETM.AI, USDM Life Sciences’ groundbreaking solution built on Oracle Guided Learning, transforms reactive compliance into proactive, AI-powered guidance embedded directly in business workflows.
The Challenge: When Manual Compliance Meets Digital Innovation
Life sciences organizations operate in an increasingly complex environment where two powerful forces are reshaping compliance management:
- Accelerating regulatory change across global markets, driven by health authorities continuously updating requirements (e.g. global medical device UDI regulations) and business expansion into new markets
- Rapid SaaS innovation cycles, where cloud solution providers introduce new features every few months that may have compliance implications across quality system procedures, IT system functionality and configurations and SOPs
Traditional manual ETM processes—the backbone of regulatory compliance in life sciences—cannot adapt quickly enough to this pace of change. The result? Companies face higher audit risks, slower adoption of digital innovations, and escalating costs of compliance management.
ETM.AI represents a paradigm shift from “compliance as documentation” to “compliance in the flow of work”
Built on Oracle Guided Learning (OGL), ETM.AI embeds GxP-critical guidance directly into enterprise application workflows, providing real-time alerts, contextual SOP access, and regulatory references at the exact moment users need them. But ETM.AI goes far beyond traditional in-application guidance—it creates the industry’s first truly digital Enterprise Trace Matrix powered by AI agents.
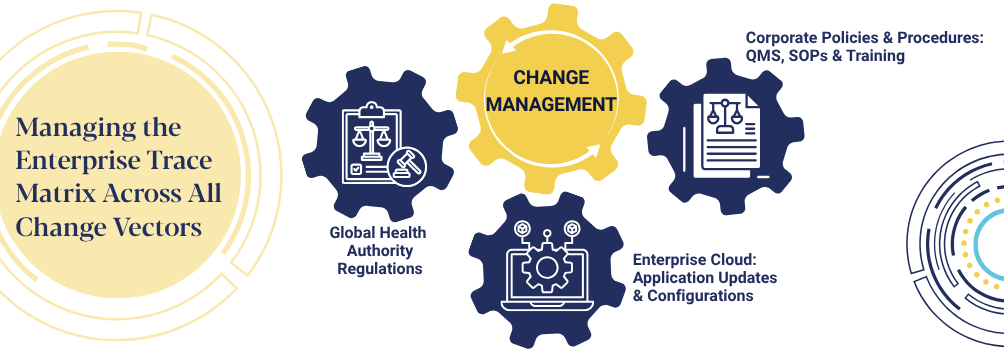
The Digital ETM: Three Pillars, One Platform
ETM.AI consolidates the three critical elements of regulatory compliance into a unified digital framework:
- Regulatory Requirements: Current regulations from FDA, EMA, and other global health authorities
- Quality Management System: SOPs, work instructions, and training certifications
- Oracle SaaS Configurations: Validated system configurations and data management controls
This consolidation creates what we call a “Localized Language Model” (LLM)—a compliance-specific AI foundation that enables Oracle AI Agents to actively manage and optimize compliance processes in real-time.
The Partnership That Makes It Possible
ETM.AI come out of USDM Life Sciences’ 20+ year strategic partnership with Oracle and our exclusive role as Oracle’s CloudSure compliance partner. As active participants in the Oracle CloudSure framework, we’ve collaborated directly with Oracle’s product teams to develop the compliance capabilities that life sciences organizations need.
This isn’t just another software integration—it’s a purpose-built solution that leverages Oracle’s enterprise platform capabilities with USDM’s deep regulatory expertise and over two decades of exclusive focus on life sciences compliance.
AI Agents: The Art of the Possible
The true power of ETM.AI lies in its AI agent capabilities, which transform static compliance frameworks into dynamic, intelligent systems:
Real-Time Compliance Management
AI agents provide contextual alerts and regulatory references at the point of task execution, ensuring users have access to the most current compliance information without leaving their workflows.
Intelligent Impact Analysis
When regulations change, your business expands into new markets, or new Oracle Cloud features are released, AI agents automatically assess the impact across all QMS processes and system configurations, identifying affected procedures and required updates.
Automated Change Control
ETM.AI’s AI agents maintain automated traceability across regulatory, system, and quality management changes, creating comprehensive audit trails that demonstrate compliance continuity.
Predictive Compliance
Perhaps most powerful of all, AI agents can identify potential compliance gaps before they become audit observations, enabling proactive remediation rather than reactive responses.
Transforming Business Outcomes
The business impact of ETM.AI extends far beyond compliance risk reduction:
- Accelerated Innovation Adoption: Companies can confidently implement new Oracle Cloud capabilities knowing their compliance framework will automatically adapt to maintain regulatory integrity.
- Operational Efficiency: One-click SOP access, embedded training, and contextual guidance eliminate the productivity losses associated with hunting for up-to-date compliance information.
- Audit Readiness: Automated documentation and comprehensive audit trails ensure organizations are always inspection-ready.
- Reduced Compliance Costs: By embedding compliance into business processes rather than treating it as a separate activity, organizations can dramatically reduce the total cost of regulatory management.
USDM’s Unique Position in Life Sciences Compliance
USDM Life Sciences brings unmatched depth to this partnership:
- 25+ years exclusively focused on life sciences compliance across pharmaceutical, biotech, and medical device industries
- Deep Oracle partnership with proven success delivering compliant cloud solutions to 200+ life sciences companies
- End-to-end support from initial process mapping through implementation, validation, and ongoing change management
The Technology Behind the Transformation
ETM.AI leverages Oracle Guided Learning’s full capabilities:
- Pop-up alerts at GxP-relevant workflow steps
- Direct links to SOPs, work instructions, and CFR references
- User acknowledgment and audit trails with optional e-signature capability
- Role-aware delivery ensuring users receive only relevant compliance guidance
- Process guides and smart tips uniquely configured for each regulated workflow
These capabilities can be extended across all Oracle SaaS solutions and can integrate with other HTML-based third-party software, providing comprehensive compliance coverage across your enterprise technology ecosystem.
Ready to Transform Your Compliance Approach?
ETM.AI represents the evolution from manual, reactive compliance management to AI-powered, proactive compliance embedded directly in business workflows. For life sciences organizations ready to innovate faster while maintaining regulatory integrity, the future of compliance management is available today.
We’ve developed proof-of-concept demonstrations showcasing ETM.AI’s capabilities and have implementation roadmaps ready for organizations prepared to modernize their compliance approach. Our solution doesn’t just address today’s compliance challenges—it positions companies to thrive in an increasingly complex regulatory environment while accelerating their adoption of digital innovation.
The question isn’t whether life sciences compliance will become AI-powered and workflow-embedded—it’s whether your organization will lead this transformation or follow others who move first.
Ready to learn more about ETM.AI and how it can transform your organization’s compliance approach? Contact USDM Life Sciences to schedule a demonstration and discuss your implementation roadmap.
John Danese, VP of Digital Innovation, USDM Life Sciences
📞 Office: 805.430.2025
📧 Email: jdanese@usdm.com
🔗 LinkedIn: linkedin.com/in/johndanese