
Leveraging AI for Enhanced Clinical Trial Data Management in Life Sciences
Discover how AI enhances clinical trial data accuracy, saves $200K annually, reduces manual effort by 80%, and accelerates timelines by 25%.
|
WHITE PAPER: An AI Use Case Dossier on Workflows, Platforms, and Operational Gains
|
Watch USDM’s AI presentation at the Veeva Summit 2024.
Discover how USDM helps to forecast patient visit statuses, streamline data entry, and optimize data cleaning processes with AI-driven predictive analytics. Learn how it helps your organization accelerate database lock, enhance data accuracy, and significantly reduce timelines for faster data-driven decisions.
This is just one use case. Imagine the possibilities! Identify a use case you want to prove and we’ll make it happen in days, not months.
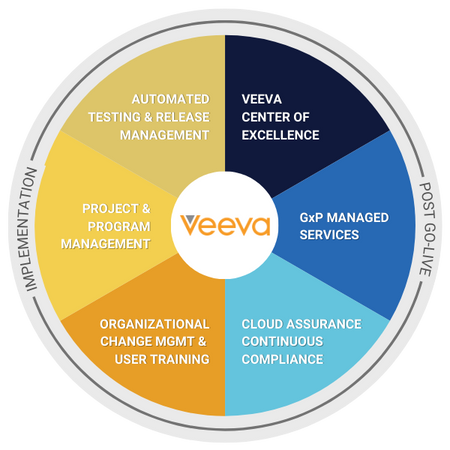
By employing best practices in content governance, standard operating procedures (SOPs), organizational change management, and post-go-live support, USDM empowers your life sciences organization to simplify regulated processes and compliance and achieve long-term success with your Veeva investments. Download our Veeva datasheet for more information.
Share your unique use cases and we’ll help you build a plan to get more value from your Veeva Vaults. Fill out this form to get started.


Discover how AI enhances clinical trial data accuracy, saves $200K annually, reduces manual effort by 80%, and accelerates timelines by 25%.

Explore ways to extract business insights from EDC metadata for query metrics, query causes, and query agents.

What is integrated GxP compliance? It’s a catalyst for innovation, adaptability, and continuous improvement while maintaining compliance of regulated cloud applications and on-premises IT systems.